Abstract

Background: Multiple myeloma (MM) remains an incurable plasma cell malignancy and predominantly affects elderly population with a median age at onset around 70 years. High-dose chemotherapy followed by autologous hematopoietic cell transplantation (HCT) remains an important consolidative measures even in the era of novel agents including anti-CD38 antibodies. HCT in elderly patients is typically met with concerns for increased toxicity in this age group. Here, we evaluated our single center experience of autologous HCT in the elderly for the last decade.
Methods: Clinical transplant data on MM patients who underwent HCT at Moffitt Cancer Center (MCC) were retrospectively reviewed. BMT Research Analysis Information Network (BRAIN) database was used for data query. The study population was limited to first autologous HCT for MM and receiving high-dose melphalan based conditioning regimens. Patients were divided into 3 groups based on their age at HCT: Age < 65 (n=1044), 65-69 (n=428), and 70+ (n=222). Overall survival (OS) and progression-free survival (PFS) were calculated using Kaplan-Meier method and compared with log-rank testing. Cumulative incidence function was compared with Gray's test.
Results: There were 1694 MM patients who underwent autologous HCT with high-dose melphalan-based regimen at MCC from 2010 to 2021. Over 100 transplants per year were performed at our institution for MM since 2011 at both inpatient and outpatient settings. Median HCT-CI was 2, 3, and 3 for ages <65, 65-69, and 70+, respectively. Median serum creatinine (range) was 0.9 (0.5-10.1), 0.8 (0.4 - 11.9), and 0.9 (0.4 - 8.4) for ages <65, 65-69, and 70+, respectively. Reduced melphalan dosing (MEL140) was used in 5.6%, 10.1%, and 82.7% for ages <65, 65-69, and 70+, respectively.
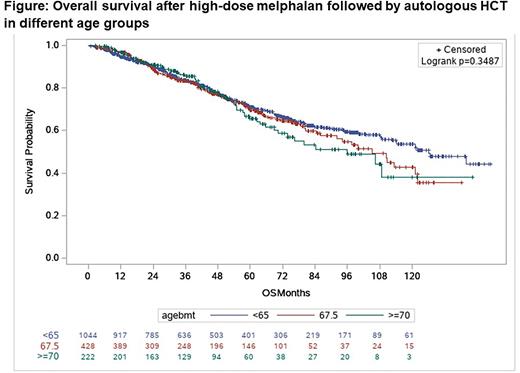
Univariate analysis showed that the 5-year OS for ages <65, 65-69, and 70+ was 71.1% (95% confidence interval (CI): 67.7-74.3), 69.6% (95%CI: 63.9-74.6), and 65.4% (95%CI: 56.4-73.0), respectively (Log rank, p=0.34, Figure). The 5-year PFS for ages <65, 65-69, and 70+ was 48.0% (95%CI: 44.3-51.5), 46.0% (95%CI: 40.0-51.8), and 46.0% (95%CI: 37.3-54.3), respectively (Log rank, p=0.64). The 5-year cumulative incidence of relapse for ages <65, 65-69, and 70+ was 44.9% (95%CI: 41.4-48.4), 44.4% (95%CI: 38.6-50.1), and 38.6% (95%CI: 31.1-46.0), respectively (Gray's test, p=0.2). The 3-moth, 1-year, and 5-year cumulative incidences of nonrelapse mortality (NRM) were 0.5% (95%CI: 0.2-1.2), 1.9% (95%CI: 1.2-2.9) and 7.0% (95%CI: 5.3-9.0) for age < 65, 0.7% (95%CI: 0.1-1.9), 0.9% (0.3-2.2%) and 9.4% (95%CI: 6.4-13.2) for ages 65-69, and 0.0% (95%CI: 0.0-0.0), 0.4% (95%CI: 0.0-2.4) and 15.2% (95%CI: 9.2-22.5) for age 70+, respectively, respectively (Gray's test, p=0.16). Additional multivariate analysis will be provided at the presentation.
Conclusions: Overall, there were no differences in the MM HCT outcomes in different age groups at our center. Our data demonstrate the safe application of high-dose therapy in MM patients across the age group. Older MM patients appear to derive comparable survival benefits from HCT and should remain a standard consolidative measure. There is a suggestion that the NRM may be numerically higher in elderly population, and further analysis on long-term outcomes in elderly population is planned.
Disclosures
Freeman:Incyte: Honoraria; Amgen: Honoraria; Janssen: Honoraria, Research Funding; Sanofi: Honoraria; Bristol Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hansen:Survivorship: Honoraria; BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; OncLive: Honoraria. Blue:Oncopeptides: Honoraria; Sanofi: Consultancy, Speakers Bureau; Jassen: Consultancy, Membership on an entity's Board of Directors or advisory committees. Grajales-Cruz:sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Liu:Sanofi: Speakers Bureau. Shain:Janssen: Honoraria, Speakers Bureau; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; GlaxoSmithKline: Speakers Bureau; Amgen: Speakers Bureau; Karyopharm: Research Funding, Speakers Bureau; AbbVie: Research Funding. Baz:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Shattuck labs: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; celgene: Consultancy, Honoraria; karyopharm: Research Funding; genentech: Membership on an entity's Board of Directors or advisory committees. Locke:Janssen: Membership on an entity's Board of Directors or advisory committees; Allogene: Research Funding; Kite Pharma: Research Funding; BMS: Research Funding; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Research Funding; Allogene: Membership on an entity's Board of Directors or advisory committees; Umoja: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BlueBird Bio: Research Funding; Calibr: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Iovance: Membership on an entity's Board of Directors or advisory committees; Clinical Care Options Oncology: Other: Education or editorial role; Imedex: Other: Education or editorial role; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Cowen: Consultancy; Novartis: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees; EcoR1: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Education or editorial role; ASH: Other: Education or editorial role; Aptitude Health: Other: Education or editorial role; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Society for Immunotherapy of Cancer: Other: Education or editorial role; A2: Membership on an entity's Board of Directors or advisory committees; National Cancer Institute: Research Funding; Emerging Therapy Solutions: Consultancy; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; Gerson Lehrman Group: Consultancy. Alsina:BMS: Research Funding; BMS, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal